- Product : IYPOLY
- Item : A dressing, fluid-impermeable film
- Model : IYPOLY NASAL SPRAY
- Ingredients : Egg Yolk Extract(IgY), Purified water, Etc.
- Origin(Main ingredient) : Japan OMR/DDS (IgY)
- Uses : Prevents penetration of the respiratory infectious viruses and protects nasal mucosa.
- NIDS Product License: 21-5026
- U.S. FDA NDC No. : 82462-103-01
- Size (Package box) : (W) 3.5 x (D) 3.5 x (H) 12.0cm
- Volume : 20ml
- DirectionsSpray 2~3 times every 8 hours into the nose and mouth. In case of showing signs of infection or if already infected, spray several times a day.

- Test Lab. : Wide River Institute of Immunology of Seoul National University / Genome Research Institute of Tokyo University of Science
- R&D : OMR (Oder-made Medical Research Inc.)
- Manufacture : WHONIZ Co.,Ltd.
- Head Office : (06307) 6F, 224, Gaepo-ro, Gangnam-gu, Seoul, Republic of Korea / Tel: +82-2-6952-6767
IYPOLY NASAL SPRAY Certificate
Click on the photo to learn more.
- Certificate of Manufacturer – certified by Korea Ministry of Food and Drug Safety (KFDA)
- Certificate of Free Sales – certified by National Institute of Medical Device Safety Information (NIDS)
- ISO 13485:2016 Medical Devices Quality Management System
- Certificate of FDA OTC registration
- CE Certificate (Council Directive 93/42/EEC regulation 4/2009)
- Certificate of Korea Halal – cross-certified with JAKIM (Malaysia Halal)
- UAE Import License
- UAE Certificate of Halal (Crystal SnR)
How to use IYPOLY
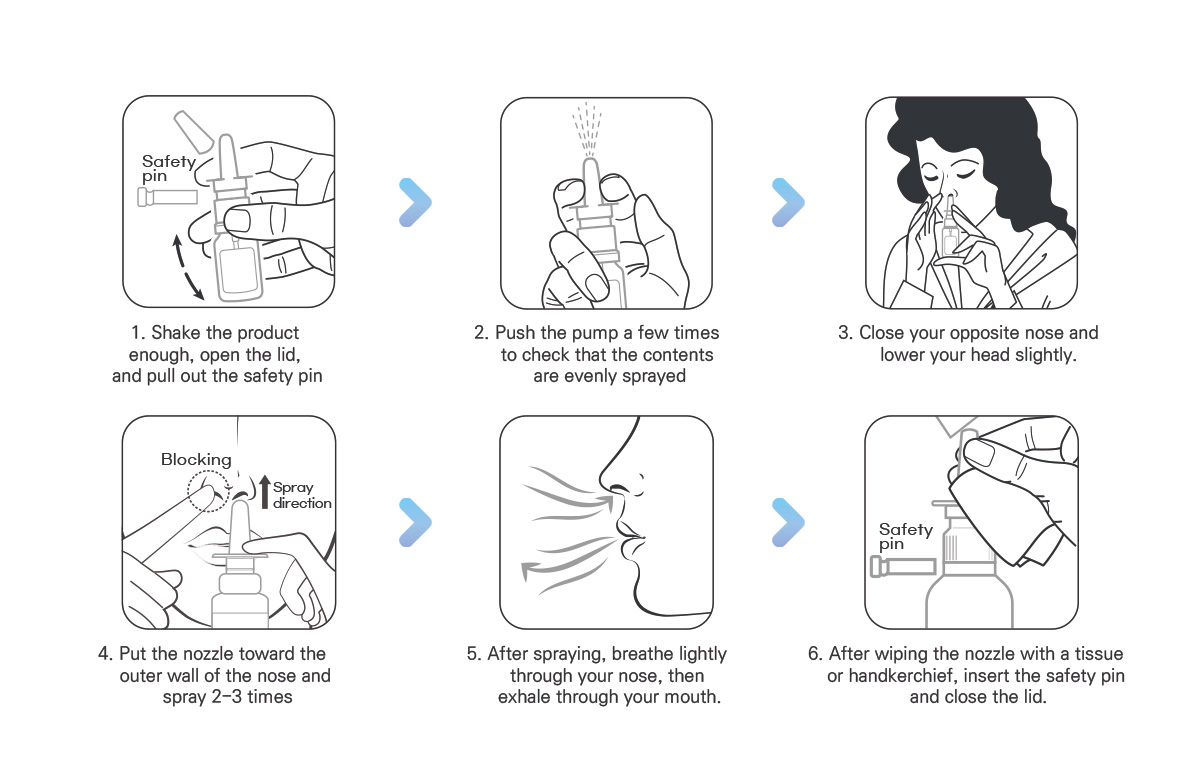
【Precautions】
- Limit the use when hypersensitivity reactions occur due to the ingredients contained in this product.
- If symptoms do not improve after using this product, consult a physician.
- For hygienic reasons and to prevent the transmission of pathogens, do not share the bottle, keep it clean and close the lid after use.
- Keep out of the reach of children.
- Avoid the direct sunlight and store in a cool place.
Photo of IYPOLY
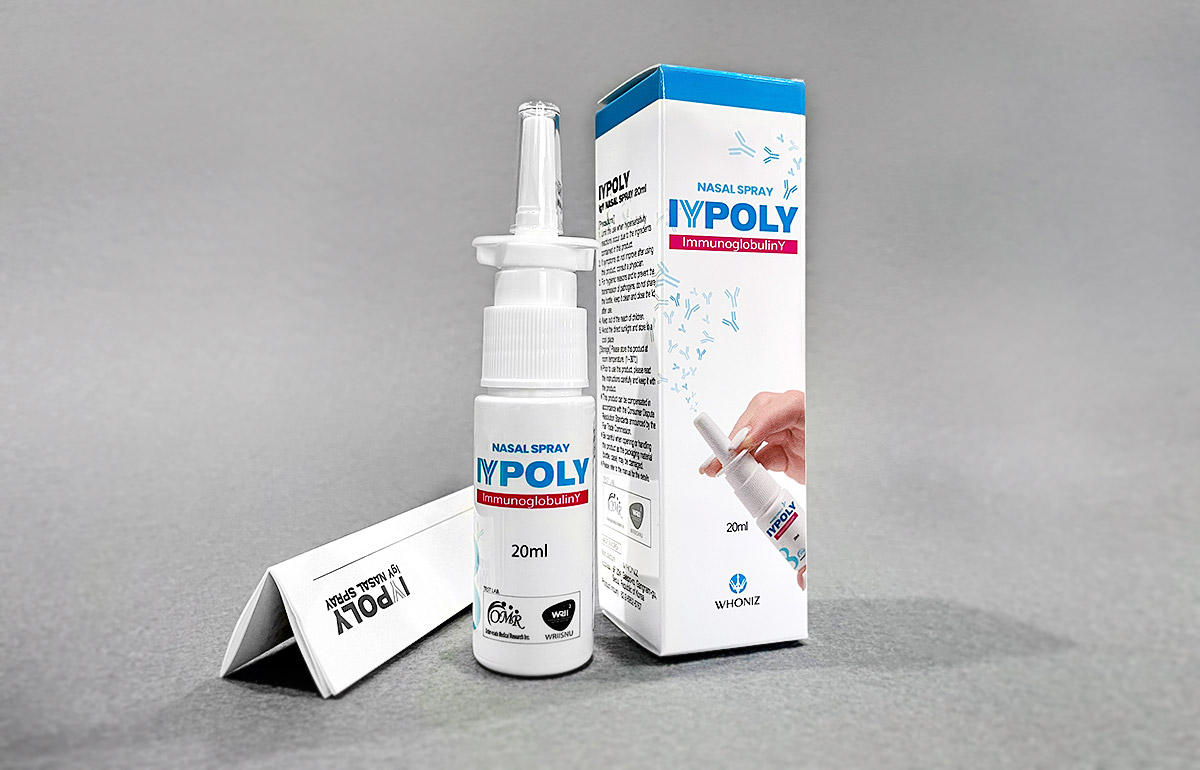
This product is for an export sales.
IYPOLY NASAL SPRAY Certificate
Click on the photo to learn more.
- Certificate of Manufacturer – certified by Korea Ministry of Food and Drug Safety (KFDA)
- Certificate of Free Sales – certified by National Institute of Medical Device Safety Information (NIDS)
- ISO 13485:2016 Medical Devices Quality Management System
- Certificate of FDA OTC registration
- CE Certificate (Council Directive 93/42/EEC regulation 4/2009)
- Certificate of Korea Halal – cross-certified with JAKIM (Malaysia Halal)
- UAE Import License
- UAE Certificate of Halal (Crystal SnR)
How to use IYPOLY

【Precautions】
- Limit the use when hypersensitivity reactions occur due to the ingredients contained in this product.
- If symptoms do not improve after using this product, consult a physician.
- For hygienic reasons and to prevent the transmission of pathogens, do not share the bottle, keep it clean and close the lid after use.
- Keep out of the reach of children.
- Avoid the direct sunlight and store in a cool place.
Photo of IYPOLY

This product is for an export sales.
Anchor product (To be developed)
Polyclonal IgY-based Preventive agents
IYPOLY Plus
- Disease : SARS-CoV-2
- Antibody : IgY (Polyclonal)
- Product : Nasal spray
- Remark : Preventive agents
This product is currently under development.

IYPOLY +A
- Disease : ADE caused by SARS-CoV-2
- Antibody : IgY (Polyclonal)
- Product : Nasal spray
- Remark : Preventive agents
This product is currently under development.
IYPOLY +M
- Disease : Monkeypox
- Antibody : IgY (Polyclonal)
- Product : Nasal spray
- Remark : Preventive agents
This product is currently under development.
IYPOLY +R
- Disease : Human Rota virus
- Antibody : IgY (Polyclonal)
- Product : Nasal Spray
- Remark : Preventive agents, therapeutics
This product is currently under development.
Monoclonal IgY-based Preventive agents
Anti-Colorectal cancer drug
- Disease : Colorectal Cancer
- Antibody : OMR- C001 (Monoclonal)
- Product : Pill, Others
- Remark : Therapeutics
This product is currently under development.

Anti-Lung cancer drug
- Disease : Lung Cancer
- Antibody : OMR- VEGF (Monoclonal)
- Product : Injection, Others
- Remark : Therapeutics
This product is currently under development.
Anchor product (To be developed)
Polyclonal IgY-based Preventive agents
IYPOLY Plus
- Disease : SARS-CoV-2
- Antibody : IgY (Polyclonal)
- Product : Nasal spray
- Remark : Preventive agents
This product is currently under development.

IYPOLY +A
- Disease : ADE caused by SARS-CoV-2
- Antibody : IgY (Polyclonal)
- Product : Nasal spray
- Remark : Preventive agents
This product is currently under development.

IYPOLY +M
- Disease : Monkeypox
- Antibody : IgY (Polyclonal)
- Product : Nasal spray
- Remark : Preventive agents
This product is currently under development.

IYPOLY +R
- Disease : Human Rota virus
- Antibody : IgY (Polyclonal)
- Product : Nasal Spray
- Remark : Preventive agents, therapeutics
This product is currently under development.

Monoclonal IgY-based Preventive agents
Anti-Colorectal cancer drug
- Disease : Colorectal Cancer
- Antibody : OMR- C001 (Monoclonal)
- Product : Pill, Others
- Remark : Therapeutics
This product is currently under development.

Anti-Lung cancer drug
- Disease : Lung Cancer
- Antibody : OMR- VEGF (Monoclonal)
- Product : Injection, Others
- Remark : Therapeutics
This product is currently under development.

 한국어
한국어 English
English 中文
中文






